Women’s health cannot wait
For too long, women have been forced to make decisions about their health care without the information they need.

At a Foreign Policy event alongside the 79th United Nations General Assembly, BRIDGE Co-Chairs Ngawai Moss and Marie Teil joined Papa Seck (U.N. Women) to discuss women’s inclusion in data and research.
By: Ngawai Moss, Melissa Tassinari, and Marie Teil
For everyone who is pregnant, there are many questions. When women living with a chronic disease – such as diabetes, epilepsy or an autoimmune condition – decide to start a family, they face more than the usual anxieties. For these women, decisions about managing their disease carry risks both for themselves and their unborn babies. However, in many cases, there are no easy answers because the data needed to provide clear guidance simply doesn’t exist – a consequence of not including pregnant and breastfeeding women in medical research.
Historically, women were excluded from clinical trials. This exclusion stemmed from biases in the design of clinical trials and a prevailing notion that it was safer to ‘protect’ pregnant and breastfeeding women by not including them. It wasn’t until the 1990’s that Congress mandated that the NIH include women in clinical research, marking a significant turning point in the rights of women to participate in studies impacting their health.
However, even today, research approaches continue to be designed around males (both humans and animals), leaving women still underrepresented in data on the effects of medicines and vaccines. These gaps are particularly stark for women who are pregnant and breastfeeding – consider the early trials of the COVID-19 vaccine, which excluded pregnant women, despite the virus disproportionately affecting health care workers who are usually women of childbearing age.
The lack of information on a drug’s effectiveness during a pregnancy or on potential risks to the developing fetus is a serious concern for any woman who takes medication. Studies report that more than 90% of pregnant women take a prescription or over-the-counter drug. Yet, despite these statistics, clinical trials for drugs treating chronic conditions have not included women along different stages of their reproductive journey. In fact, many clinical trials require participating women to take contraception or withdraw from the trial if they become pregnant. For a woman with a chronic condition, regular use of medication is needed to maintain her health. As a result, women living with chronic diseases are often left without a clear understanding of the benefits and/or possible risks of continuing or stopping their medications.
It is unacceptable that women – and their health providers – are forced to make critical decisions without the proper tools, resources, or data to make fully informed choices. We need to change the paradigm to ensure that every woman’s right to health information is guaranteed.
This is why we joined forces to create the BRIDGE Commission. BRIDGE is a global, multidisciplinary group of patients, women’s health advocates, health care professionals, ethicists, and researchers. Together, we are working to ensure that women with chronic diseases are equipped with reliable, high-quality data to make informed decisions about their treatment, from the time of diagnosis of a chronic condition through all phases of their reproductive health journey.
The report we published earlier this year calls for a change in the global mindset. We want to move from a place of fear and “protecting” a pregnant or breastfeeding woman – and her fetus or newborn – to a thoughtful, safe and inclusive approach that enables her participation in research, generating the much-needed data that women of reproductive age deserve.
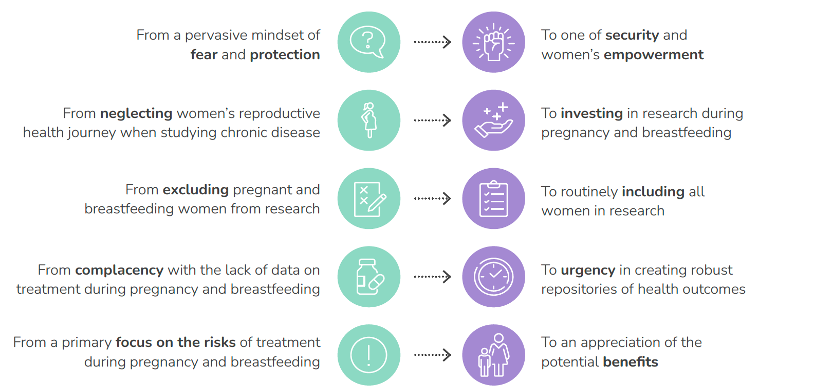
Many stakeholders have important roles to play in changing the paradigm. In the future this would mean:
· Starting in the doctor’s office, health care providers have early discussions with their patients about their reproductive journey.
· Industry and academic researchers plan to involve women throughout the research process to answer questions women care about.
· Policymakers require that research studies evaluate the potential impact on pregnant and breastfeeding women for every drug in development.
· Health systems must continuously update care protocols to reflect the latest evidence-based guidance.
· Patient advocacy groups elevate the awareness of data gaps that affect women living with a chronic disease and advocate for data generation.
· Medical journals and consumer media place the risks of treatment in context to convey both benefits and risks of continuing treatment throughout pregnancy and breastfeeding.
On the global stage, there is growing recognition that our approach to women’s health research needs to change. Over the past year, the United States government has begun to develop new policies to drive women’s health research and innovation and the World Economic Forum launched the Global Alliance for Women’s Health, a multi-sector platform to prioritize, protect, and promote the health of women. The conversation about women’s health has also included important discussions around gender equity, financial inclusion for women, and female political leadership alongside the 79th United Nations General Assembly.
It is long past time for greater representation of women in research, particularly during their reproductive health journey. We believe that access to relevant, evidence-based health information is every woman’s fundamental right. Women deserve to be at the forefront of decision-making about their health – and we each have a part to play in ensuring this future becomes a reality. We invite you join us in this effort.
Ngawai Moss, Melissa Tassinari, and Marie Teil are Co-Chairs of the BRIDGE Commission.
Ngawai Moss is a Women’s Health and Research Advocate, Honorary Research Fellow at Queen Mary University of London, and Founding Member of UK-based Elly Charity.
Melissa Tassinari is a Former Clinical Advisor at FDA.
Marie Teil is the Vice President of Special Patient Populations at UCB Biopharma.
